Published online Dec 24, 2015. doi: 10.5500/wjt.v5.i4.348
Peer-review started: June 1, 2015
First decision: August 14, 2015
Revised: September 2, 2015
Accepted: October 12, 2015
Article in press: October 13, 2015
Published online: December 24, 2015
Processing time: 209 Days and 6.9 Hours
AIM: To perform a systematic review of literature on use of cardiovascular imaging in assessment of donor hearts.
METHODS: A systematic search of current literature from January 1965 to August 2015 was performed using PubMed and Google Scholar to investigate the different imaging modalities used to assess donor hearts.
RESULTS: Recent literature still estimates only a 32% utilization of available donor hearts in the United States. Most common imaging modality used is transthoracic echocardiography. Use of advanced imaging modalities such as 3D echocardiography, cardiac computer tomography and cardiac magnetic resonance to evaluate donor hearts is not reported in literature. This review attempts to highlight the relevant imaging modalities that can be used to assess cardiac function in a time-efficient manner. The algorithm suggested in this review would hopefully pave the way to standardized protocols that can be adopted by organ procuring organizations to increase the donor pool.
CONCLUSION: Use of advanced imaging techniques for a thorough assessment of organs will likely increase the donor pool.
Core tip: The increase in the number of patients on the cardiac transplant waiting list unfortunately has not been accompanied by a concomitant increase in the number of donor organs. In the present era of advanced imaging techniques it is imperative to use them for a thorough assessment of organs before they are deemed unfit for use. Three-dimensional echocardiography and cardiac magnetic resonance imaging are powerful techniques that could be used for assessing hearts that do not pass the standard tests. This review highlights potential imaging techniques that can be used to assess donor hearts for better utilization of organs.
- Citation: Nair N, Gongora E. Role of cardiovascular imaging in selection of donor hearts. World J Transplant 2015; 5(4): 348-353
- URL: https://www.wjgnet.com/2220-3230/full/v5/i4/348.htm
- DOI: https://dx.doi.org/10.5500/wjt.v5.i4.348
Increasing numbers of advanced heart failure patients on the transplant waiting list and the dwindling availability of the donor pool should prompt methods of improving donor heart selection so that the “marginal hearts” can be assessed and utilized effectively. This review addresses the role of advanced cardiovascular imaging in donor heart selection. With the advent of more powerful imaging techniques such as real time 3D echocardiography and cardiac magnetic resonance imaging organ screening should become more efficient if these techniques are used in a systematic fashion. In a recent retrospective analysis of the United Network of Organ Sharing database Khush et al[1] showed that the percentage of donor hearts accepted for transplant decreased from 44% in 1995 to 29% in 2006 with an increase to 32% in 2010. Though increase in rejection rate of donor hearts has been based on age and co morbidities there are no evidence - based guidelines to support this. Hence efforts in this direction would be helpful[1]. In another retrospective investigation only two statistically relevant causes such as death and history of diabetes have been implicated in prolonged post-operative hospital stay and increased mortality respectively[2].
Echocardiography has been used in selection of donor hearts since the last three decades[3]. In a recent study 25% to 50% of hearts have been reported to be rejected due to echocardiographic abnormalities. Statistically significant variation in interpretation of echocardiographic data [left ventricular internal dimension at diastole (LVIDd), left ventricular internal dimension at systole (LVIDs) and left ventricular ejection fraction (LVEF)] was noted in a retrospective study[4]. Difficulty in obtaining adequate imaging adds to the problem. Contrast echocardiography has been suggested to improve imaging[5]. However present day advanced imaging modalities are far less utilized in the donor selection process. It is therefore relevant to assess use of new modalities of cardiovascular imaging in donor heart selection to avoid discarding hearts that have been inadequately imaged due to technique or patient characteristics. Such an approach may increase utilization of the presently discarded organ pool.
Searches were conducted from January 1965 to August 2015 in the PubMed and Google Scholar databases using the terms “selection of donor hearts” retrieved 1002 articles. Using the term “imaging in donor hearts” showed 311 articles and further narrowing the search to “imaging in selection of donor hearts” retrieved 9 articles. This review was planned to be a qualitative overview hence no statistical analyses were performed.
The results from the searches conducted are summarized in this section highlighting the use of various cardiovascular imaging techniques to assess selection of donor hearts.
Pharmacological stress echocardiography: Pharmacological stress echocardiography appears to be an attractive option to test the suitability of donor hearts which would not meet standard criteria. Low dose dobutamine was shown to be useful in assessing hearts from brain dead donors over a decade ago[6]. In a more recent series of papers from Europe stress echocardiographic screening appears to be useful in increasing the marginal donor pool and also have a reasonable outcome in the post-transplant patients[7-11]. Stress echo studies can efficiently differentiate hearts that have subclinical coronary artery disease or cardiomyopathy[7-12]. Besides, in patients with normal valve function, stress echo coupled with tissue doppler imaging can be used to assess diastolic dysfunction[13]. Another advantage of using stress echo is that it can detect cardiomyopathy and global ventricular dysfunction secondary to causes other than epicardial coronary artery disease. In older populations of donors, diabetes and/hypertension may coexist and contribute to subclinical disease[13]. Therefore, a complete assessment of systolic and diastolic function can be obtained non-invasively in a time effective manner.
Current reports in literature support successful use of stress echocardiography in populations of donors with reversible left ventricular (LV) dysfunction as well as those with stunned hearts that improve with hormonal treatment. As part of the Adonhers (aged donor heart rescue by stress echo) project 43 recipients who received “marginal” hearts and were older than 55 years of age or had concomitant risk factors were followed for 3 years. The outcomes in these recipients were unremarkable with a 1 year survival of 93% suggesting a role for stress echo screening of donor hearts to increase the donor pool[11] .
One of the limitations of this approach is that long term outcomes have not been studied yet[11]. The pharmacological agents used currently are dipyridamole and dobutamine with the latter being less preferred due to high heart rates in the resting state in the donor hearts secondary to the high catecholamine state[11].
Strain rate imaging: The principle of strain and strain rate imaging based on myocardial deformation is an emerging technique which can be useful in donor heart evaluations. It has been shown to be effective in distinguishing ischemic from stunned myocardium and also in the early detection of cardiomyopathies in the setting of a normal ejection fraction[14-17]. Myocardial deformation imaging can be achieved by tissue doppler imaging as well as speckle tracking. The use of strain and strain rate imaging by speckle tracking is better than velocity/displacement measurements because speckle tracking can distinguish active vs passive myocardial tissue movements. Strain and strain rate imaging (SRI) can be directly obtained using pulsed wave tissue Doppler (PW-TDI) or reconstructed from color tissue Doppler imaging (c-TDI). These methods are currently well accepted as tools to investigate regional and global cardiac function[16-18]. Non-Doppler 2D-strain imaging using speckle tracking analyzes motion by tracking speckles from frame to frame. The change in speckle position is used to determine its velocity. Since tracking is done in 2 dimensions it is angle independent. Speckle tracking is also time efficient as compared to TDI-strain imaging but needs high image quality which may present a problem in patients who are technically difficult to image. However, good correlation exists between the SRI done with TDI as well as non-doppler 2-dimensional imaging[14,19,20]. The concept of SRI is attractive and can be more powerful if used in the 3D format though this needs further investigation. SRI has been used in a wide variety of clinical applications including detection of cardiac allograft rejection[21-23]. SRI has the potential to become an important tool that can be added to the regimen of non-invasive techniques used to assess donor hearts because myocardial dysfunction can be detected even in the setting of normal ejection fraction. Such studies will also open avenues for further research to develop robust imaging protocols for rapid screening of donor hearts.
Contrast enhanced 2D and 3D echocardiography: Contrast echocardiography can be used to better define the endocardium in patients whose ejection fraction is ambiguous due to this particular reason. In recent studies including a systematic review and meta-analysis 3D echocardiography (3DE) was found to underestimate LV volumes and LVEF and was also useful only in patients with good acoustic windows and normal sized ventricles. Large variations in determinations were noted in populations with poor images and enlarged ventricles. With acceptable image quality 3DE is more accurate and precise in measuring EF and LV volumes than 2DE. As compared to cardiac magnetic resonance imaging (MRI), 3DE is inferior in spatial and temporal resolution[24]. In another retrospective review of literature both contrast 2DE and non-contrast 3DE had similar agreement with cardiac MRI. Contrast 3DE needs further evaluation because non-contrast 3DE is useful only in patients with optimal images[25]. A prospective study by Jenkins et al[26] in 2009 in 60 patients with a history of myocardial infarction showed that when compared with cardiac MRI, contrast 2DE and non - contrast 3DE were similar. The best agreements with cardiac MRI were obtained in this population of patients with contrast 3DE. Contrast 3DE may be useful in patients with poor imaging windows but needs further research studies in cardiomyopathies of different etiologies. This technique could also be useful in assessing donor heart which is otherwise poorly visualized.
Cardiac MRI: The use of cardiac MRI in mechanically ventilated patients has been demonstrated to be safe in a number of studies in the adult and pediatric populations. Children typically require sedation. Hence mechanical ventilation under general anesthesia eliminates motion artifacts and eliminates the need for breath holding. In a small study done in infants on high-frequency oscillatory ventilation showed no adverse effects as compared to controls[27]. The effect of positive pressure ventilation on the cardiac output and cardiac volumes have been found to be significant in agreement with the Frank-Starling law[28] and this must be taken into consideration while evaluating donor hearts by cardiac MRI. Considering the versatility of cardiac magnetic resonance imaging (CMRI) in assessing cardiac function and its feasibility in mechanically ventilated patients cardiac MRI protocols need to be instituted and actively used in evaluating donor hearts. This would help increase the donor pool and efficiently utilize organs for transplantation. Cardiac MRI would be invaluable in providing endocardial and structural definition in assessing donor hearts.
Determination of cardiac function by computed tomography: In the process of acquiring images for coronary angiography cardiac computed tomography (CT) can be used to determine structural information to compute ventricular volumes and ejection fraction. Electron beam CT with high temporal resolution can be used to determine chamber function. Multi detector CT with better spatial but lower temporal resolution is also another modality to assess ejection fraction and obtain structural data[29]. A newer protocol with low dose radiation using a 64 slice cardiac CT technology has been recently demonstrated to be successful in determining LV ejection fraction in a small study[30]. However the large amount of contrast dye used in these modalities and the lower heart rates required may be a limitation in donor evaluations.
Optimal assessment of LV and right ventricular function: The chamber quantification and derivation of LV ejection fraction should be obtained by the biplane method of disks (modified Simpson’s rule)[31]. In patients with good imaging windows 3DE should be used if the instrumentation is available on site[31]. Global longitudinal strain should be measured from three standard apical views and values used to arrive at the average[31]. A thorough assessment of right ventricular (RV) function should be performed especially in donors who happen to be victims of motor vehicle trauma. The complete assessment of valvular function is imperative to enable any repairs that may need to be performed before transplant. Such measures would help salvage “marginal” hearts.
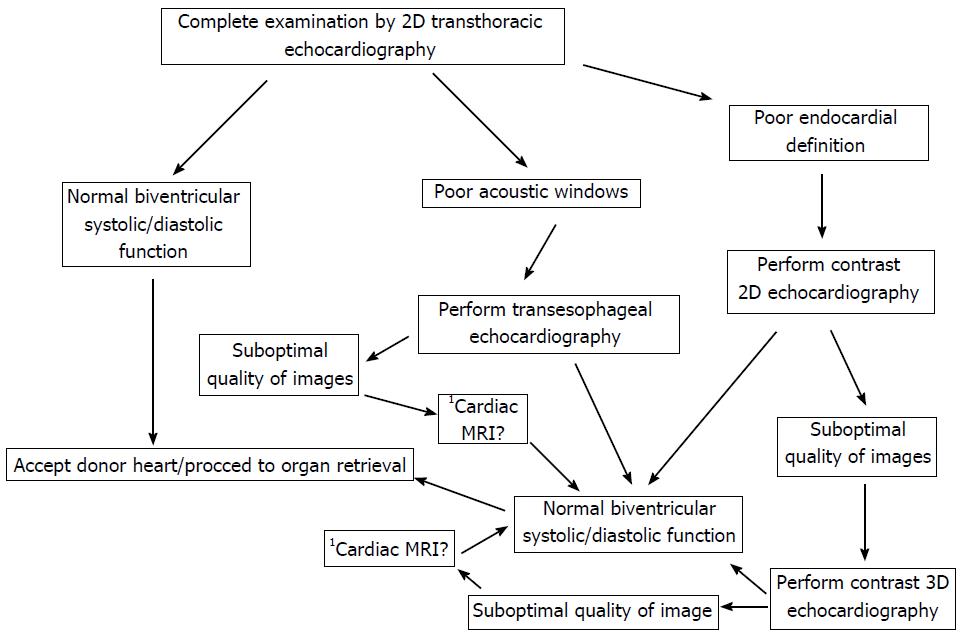
At present utilization of the donor pool of hearts is suboptimal. Increasing use of non - invasive cardiovascular imaging modalities to risk stratify the use of marginal hearts could provide a solution to increase the donor pool and therefore decrease the shortage of donor organs. 2D echocardiography could continue to be the first line imaging modality but advanced techniques should be used before a decision is made to discard the donor heart. Use of advanced imaging could also help in identifying subclinical disease which could potentially destroy graft survival. Figure 1 of this review shows a suggested algorithm to improve donor heart utilization by incorporating currently available cardiovascular imaging techniques. Table 1 shows a suggested list of parameters to be evaluated to assess RV and LV function based on the latest guidelines on chamber quantification as well as assessment of left and right ventricular function[31,32]. Hospitals will have to collaborate with organ procuring organizations to optimize protocols for better utilization of the donor organ pool. This would be very important as all hospitals may not have the complete spectrum of advanced imaging techniques.
| Parameters assessed for RV and LV function |
| Left ventricle |
| Ejection fraction |
| Wall motion score index |
| Assessment of aortic and mitral valves |
| Assessment of disatolic function by tissue doppler |
| Obtain Ea, E/A, E/Ea |
| Assessment of pulmonary vein flows |
| Assessment of longitudinal strain |
| Right ventricle |
| Fractional area change |
| Assessment of tricuspid and pulmonary valves |
| Estimation of pulmonary hypertension |
| Assessment of longitudinal strain |
| Assessment of TAPSE |
This review highlights the availability of an extensive array of cardiovascular imaging techniques which can be utilized to assess donor heart function so that more organs can be made available for cardiac transplantation. With the advent of present day technologies it is imperative that we utilize all available techniques to assess the donor hearts before they are discarded. It should also be noted that any one technique may not be adequate for a complete definitive examination. Though advanced technologies such as cardiac magnetic resonance imaging may not be readily available in all hospitals efforts must be made by organ procuring organizations to coordinate with larger hospitals and institute protocols so that “marginal hearts” can be salvaged. In combination with coronary angiography and right heart catheterization an advanced imaging approach may open up the way for better utilization of the donor pool.
Advances in cardiovascular imaging in the last two decades have been exponential. Powerful non-invasive techniques are now becoming available to delineate cardiac structure and correlate with function very precisely. This review therefore highlights the potential utility of these technological advances in selection of donor hearts. In the United States only about one third of the donor heart pool is used for cardiac transplantation. Hence improvement in assessment modalities will refine the selection process and increase the use of donor organs. The primary aim of this review is to discuss the utility of present day cardiovascular imaging in selection of organs that do not pass the standard criteria and how this can affect better utilization of the available donor pool which is far less as compared to the need for donor hearts for patients actively waiting in the cardiac transplant waiting list.
Since the first heart transplant in 1967 a number of advancements have occurred in the field of cardiac transplantation which has improved the survival of patients. The most notable ones include the discovery of cyclosporine for immunosuppression. Today cardiac transplantation still remains the gold standard for end stage heart failure. However the numbers of donor hearts that are used for transplantation are far less than the number of patients on the cardiac waiting list. One of the ways to improve increased utilization of donor hearts is to use all the different advanced imaging techniques currently available especially cardiac magnetic resonance as well as strain rate imaging and 3D echocardiography to assess structure and function prior to organ harvest. Hence an algorithm developed to utilize advanced techniques would be valuable for better use of the donor pool in the face of severe organ shortage.
The use of advanced imaging techniques to better utilize donor hearts would be an important area of investigation. In the authors’ review of the existing literature there are no investigations using cardiac magnetic resonance imaging (MRI) prior to harvesting of the donor heart. Cardiac MRI and other powerful non-invasive tests such as strain imaging, 3D echocardiography and cardiac computed tomography (CT) are not utilized to review hearts prior to harvesting. These techniques are used infrequently to study post transplant hearts. Therefore, systematic studies to prove the utility of these techniques in assessing the donor pool are warranted.
This review was undertaken to assess the extent of use of advanced cardiac imaging in the process of procuring hearts. From the current literature it is evident that these modalities are under - utilized. Hence an algorithm has been suggested in this review for use of echocardiography, cardiac MRI and other advanced modalities to increase the donor organ supply appropriately.
All the current advanced cardiac imaging modalities such as echocardiography, cardiac MRI and cardiac CT have been adequately described in this review with reference to their suitability in selecting hearts for cardiac transplantation. Each technique has been described in detail to include the current advancements.
The review presented here attempts to address the use of advanced cardiovascular imaging techniques in improving utilization of the donor pool of hearts to reduce organ shortage and waiting times for the patients which is a major limiting factor in the field of cardiac transplantation.
P- Reviewer: Takebayashi S S- Editor: Tian YL
L- Editor: A E- Editor: Li D
| 1. | Khush KK, Zaroff JG, Nguyen J, Menza R, Goldstein BA. National decline in donor heart utilization with regional variability: 1995-2010. Am J Transplant. 2015;15:642-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 2. | Khush KK, Menza R, Nguyen J, Zaroff JG, Goldstein BA. Donor predictors of allograft use and recipient outcomes after heart transplantation. Circ Heart Fail. 2013;6:300-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 3. | Gilbert EM, Krueger SK, Murray JL, Renlund DG, O’Connell JB, Gay WA, Bristow MR. Echocardiographic evaluation of potential cardiac transplant donors. J Thorac Cardiovasc Surg. 1988;95:1003-1007. [PubMed] |
| 4. | Khush KK, Nguyen J, Goldstein BA, McGlothlin DP, Zaroff JG. Reliability of transthoracic echocardiogram interpretation in potential adult heart transplant donors. J Heart Lung Transplant. 2015;34:266-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Bhatia VK, Senior R. Contrast echocardiography: evidence for clinical use. J Am Soc Echocardiogr. 2008;21:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Kono T, Nishina T, Morita H, Hirota Y, Kawamura K, Fujiwara A. Usefulness of low-dose dobutamine stress echocardiography for evaluating reversibility of brain death-induced myocardial dysfunction. Am J Cardiol. 1999;84:578-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Leone O, Gherardi S, Targa L, Pasanisi E, Mikus P, Tanganelli P, Maccherini M, Arpesella G, Picano E, Bombardini T. Stress echocardiography as a gatekeeper to donation in aged marginal donor hearts: anatomic and pathologic correlations of abnormal stress echocardiography results. J Heart Lung Transplant. 2009;28:1141-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Bombardini T, Gherardi S, Arpesella G, Maccherini M, Serra W, Magnani G, Del Bene R, Picano E. Favorable short-term outcome of transplanted hearts selected from marginal donors by pharmacological stress echocardiography. J Am Soc Echocardiogr. 2011;24:353-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Bombardini T, Gherardi S, Leone O, Sicari R, Picano E. Transplant of stunned donor hearts rescued by pharmacological stress echocardiography: a “proof of concept” report. Cardiovasc Ultrasound. 2013;11:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Franchi D, Cini D, Arpesella G, Gherardi S, Calamai I, Barletta G, Valente S, Pasanisi E, Sansoni S, Ricci C. Second-opinion stress tele-echocardiography for the Adonhers (Aged donor heart rescue by stress echo) project. Cardiovasc Ultrasound. 2010;8:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Bombardini T, Arpesella G, Maccherini M, Procaccio F, Potena L, Bernazzali S, Leone O, Picano E. Medium-term outcome of recipients of marginal donor hearts selected with new stress-echocardiographic techniques over standard criteria. Cardiovasc Ultrasound. 2014;12:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Casartelli M, Bombardini T, Simion D, Gaspari MG, Procaccio F. Wait, treat and see: echocardiographic monitoring of brain-dead potential donors with stunned heart. Cardiovasc Ultrasound. 2012;10:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Picano E, Pellikka PA. Stress echo applications beyond coronary artery disease. Eur Heart J. 2014;35:1033-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Perk G, Tunick PA, Kronzon I. Non-Doppler two-dimensional strain imaging by echocardiography--from technical considerations to clinical applications. J Am Soc Echocardiogr. 2007;20:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 355] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 15. | Heimdal A, Støylen A, Torp H, Skjaerpe T. Real-time strain rate imaging of the left ventricle by ultrasound. J Am Soc Echocardiogr. 1998;11:1013-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 540] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 16. | Edvardsen T, Gerber BL, Garot J, Bluemke DA, Lima JA, Smiseth OA. Quantitative assessment of intrinsic regional myocardial deformation by Doppler strain rate echocardiography in humans: validation against three-dimensional tagged magnetic resonance imaging. Circulation. 2002;106:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 382] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 17. | D’hooge J, Heimdal A, Jamal F, Kukulski T, Bijnens B, Rademakers F, Hatle L, Suetens P, Sutherland GR. Regional strain and strain rate measurements by cardiac ultrasound: principles, implementation and limitations. Eur J Echocardiogr. 2000;1:154-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Uematsu M, Miyatake K, Tanaka N, Matsuda H, Sano A, Yamazaki N, Hirama M, Yamagishi M. Myocardial velocity gradient as a new indicator of regional left ventricular contraction: detection by a two-dimensional tissue Doppler imaging technique. J Am Coll Cardiol. 1995;26:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 226] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Marwick TH. Measurement of strain and strain rate by echocardiography: ready for prime time? J Am Coll Cardiol. 2006;47:1313-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 419] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 20. | Ingul CB, Torp H, Aase SA, Berg S, Stoylen A, Slordahl SA. Automated analysis of strain rate and strain: feasibility and clinical implications. J Am Soc Echocardiogr. 2005;18:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Dandel M, Lehmkuhl H, Knosalla C, Suramelashvili N, Hetzer R. Strain and strain rate imaging by echocardiography - basic concepts and clinical applicability. Curr Cardiol Rev. 2009;5:133-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 280] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 22. | Dandel M, Knosalla C, Lehmkuhl H, Hetzer R. Non-Doppler two-dimensional strain imaging-clinical applications. J Am Soc Echocardiogr. 2007;20:1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Park SM, Prasad A, Rihal C, Bell MR, Oh JK. Left ventricular systolic and diastolic function in patients with apical ballooning syndrome compared with patients with acute anterior ST-segment elevation myocardial infarction: a functional paradox. Mayo Clin Proc. 2009;84:514-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 24. | Dorosz JL, Lezotte DC, Weitzenkamp DA, Allen LA, Salcedo EE. Performance of 3-dimensional echocardiography in measuring left ventricular volumes and ejection fraction: a systematic review and meta-analysis. J Am Coll Cardiol. 2012;59:1799-1808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 354] [Cited by in RCA: 298] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 25. | Wood PW, Choy JB, Nanda NC, Becher H. Left ventricular ejection fraction and volumes: it depends on the imaging method. Echocardiography. 2014;31:87-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 26. | Jenkins C, Moir S, Chan J, Rakhit D, Haluska B, Marwick TH. Left ventricular volume measurement with echocardiography: a comparison of left ventricular opacification, three-dimensional echocardiography, or both with magnetic resonance imaging. Eur Heart J. 2009;30:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 194] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 27. | Chaves AH, Cava JR, Simpson P, Hoffman GM, Samyn MM. Infant cardiac magnetic resonance imaging using oscillatory ventilation: safe and effective. Pediatr Cardiol. 2013;34:1201-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Kyhl K, Ahtarovski KA, Iversen K, Thomsen C, Vejlstrup N, Engstrøm T, Madsen PL. The decrease of cardiac chamber volumes and output during positive-pressure ventilation. Am J Physiol Heart Circ Physiol. 2013;305:H1004-H1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Singh RM, Singh BM, Mehta JL. Role of cardiac CTA in estimating left ventricular volumes and ejection fraction. World J Radiol. 2014;6:669-676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Yang Y, Yam Y, Chen L, Aljizeeri A, Aliyary Ghraboghly S, Al-Harbi I, Pen A, Ruddy TD, Chow BJ. Assessment of left ventricular ejection fraction using low radiation dose computed tomography. J Nucl Cardiol. 2015;May 22; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1-39.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6446] [Cited by in RCA: 9209] [Article Influence: 920.9] [Reference Citation Analysis (0)] |
| 32. | Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685-713; quiz 786-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4670] [Cited by in RCA: 5292] [Article Influence: 352.8] [Reference Citation Analysis (0)] |